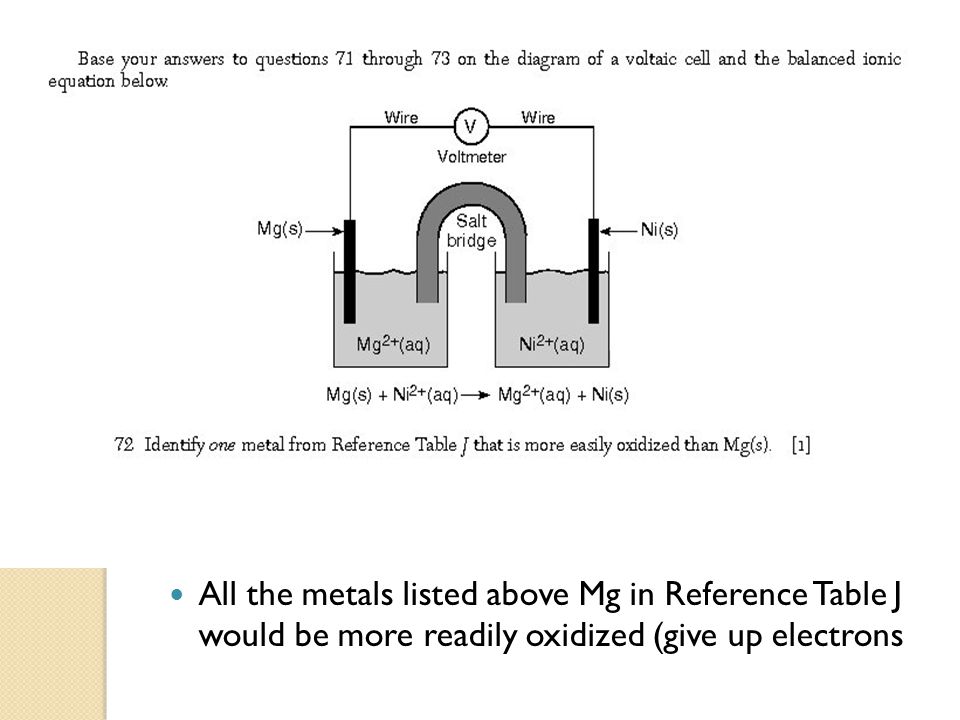
Which Metal That Is More Easily Oxidized Than Mg S
What happens in a voltaic cell. That would be the case if the metal the pure metal is more easily oxidized than hydrogen gas is.
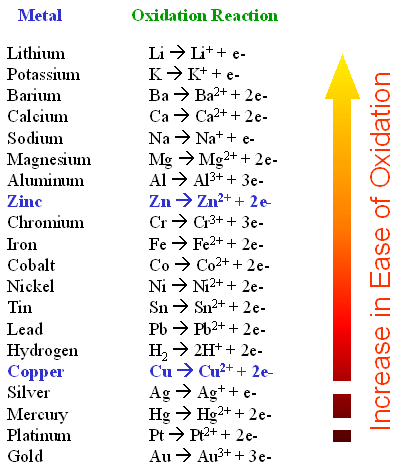
The top metals are more reactive than the metals on the bottom.
. Lithium potassium calcium sodium magnesium. 64 Identify one metal from table J that is more easily oxidized than Zn. Mg s 2 H aq H 2 g Mg 2 aq Zn s 2 H aq H 2 g.
The activity series is a chart of metals listed in order of declining relative reactivity. However different metals have different reactivities towards oxygen unreactive metals such as gold and platinum do not readily form oxides when exposed to air. Lithium potassium calcium sodium magnesium aluminum zinc chromium iron cobalt nickel lead hydrogen copper mercury silver platinum and.
Up to 24 cash back 4 Bas Which metal is more active than Ni and less active than Zn. Which metal that is more easily oxidized than Mg s. AMgs B Mg2 aq C Ags D Agaq Which species is oxidized when the switch is closed.
Metals that resist oxidation are the opposite of the metals prone to it known as the base metals. Which metal is most easily oxidized. Exposed to the oxygen in the air it catches fire.
However if we supply heat and a steady pressurized flow of H_2g we can force this reaction to occur. The order of some common metals in the electromotive series starting with the most easily oxidized is. Copper is the least easily oxidized of these particular metals.
Zinc is the next most easily oxidized. The order of some common metals in the electromotive series starting with the most easily oxidized is. A electrodes B a voltmeter C an external conductor Da salt bridge 46In a chemical cell composed of two half-cells ions are allowed to flow from one half-cell to another by means of A fusion Bredox C transmutation D cracking.
Lithium potassium calcium sodium magnesium aluminum zinc chromium iron cobalt nickel lead hydrogen copper mercury silver platinum and gold. The order of some common metals in the electromotive series starting with the most easily oxidized is. As expected these metals react with both acids and nonmetals to form ionic compounds.
Which metal is the most easily oxidized. 104 Base your answers to questions 59 through 61 on the diagram of the voltaic cell below. Why does magnesium ribbon continues to burn in carbon dioxide though it is a fire extinguisher.
Why is Fe2 more easily oxidized to Fe3 than Mn 2 to Mn 3. For example both magnesium and zinc can react with hydrogen ions to displace H 2 from a solution by the reactions. Lithium potassium calcium sodium magnesium aluminum zinc chromium iron cobalt nickel lead hydrogen copper mercury silver platinum and gold.
Explain the function of the salt bridge in the voltaic cell. When the switch is closed in which half-cell does oxidation occur. Exposed to the oxygen in the air it catches fire.
What is most likely to be oxidized. B Electrical energy is changed to chemical energy. C Electrical energy is changed to magnetic energy.
Identify one metal from Reference Table J that is more easily oxidized than Mgs. Even if that is the case. Because Magnesium like other.
Because magnesium and aluminum are higher in the series than silver both of these metals are more likely to bind with sulfide ions than silver. A Chemical energy is changed to a electrical energy. Examples of base metals include copper lead tin aluminum nickel zinc iron steel molybdenum tungsten and other transitional metals.
The element oxidized is the one whose oxidation number increased. The order of some common metals in the electromotive series starting with the most easily oxidized is. Which metal is the most easily oxidized.
The formation of hydrogen gas indicates that the heavier alkaline earth metals are better reducing agents more easily oxidized than is hydrogen. Also why are metals easily oxidized. The metals at the top of the reactivity series are powerful reducing agents since they are easily oxidized.
The electron to be removed from Fe2 is paired having charge repulsions making it easier to remove ie. Since all of these reactions involve the loss of electrons by the metal we can say that magnesium metal is the most easily oxidized. Answer-- a metal above ZnMnTi Al Mg Na Ca Sr Ba Cs K Rb Li 65 Explain in terms of Zn atoms and Zn ions why the mass of the Zn electrode decreases as the cell operates.
2 Mg 4 Pb Identify one metal from Reference Table J that is more easily oxidized than Mgs. The products of the reaction with water are hydrogen and the metal hydroxide. Two chemistry students each combine a different metal with hydrochloric acid.
Brass and bronze and the alloys of these metals also are classified as base metals. This kind of reaction may be backwards ie. Identify the pair of metals that lists the more easily oxidized metal on the left.
Of the metals that can be practically collected and handled cesium is the most easily oxidized. Any metal higher than silver on the series is more reactive is more easily oxidized and is more likely to react with sulfide ions than silver. The metals at the top of the table are most easily oxidized.
Base your answer to question 7 using the information below and your knowledge of chemistry. It may be naturally nonspontaneous. The ionization energy is smaller.
These metals tarnishcorrode very easily. Fe2 is easy to oxidize to Fe3 because ions with an odd charge are most stable for atoms with an even atomic number. Lithium sodium potassium rubidium caesium francium.
A highly active metal B moderately active metal C slightly active metal D an inactive metal 2. Of the metals that can be practically collected and handled cesium is the most easily oxidized.

Pin En Reactions And Processes
Other Oxidation Reduction Reactions

3d Printed Metal Gear Rex Metalgearsolid Mgs Mgsv Metalgear Konami Cosplay Ps4 Game Mgsvtpp Metal Gear Rex 3d Printed Metal Metal Gear
Causes Chemistry Of Rusting Rust Prevention Introduction To Oxidation Reduction Redox Reactions Gcse Igcse Ks4 Science Chemistry Revision Notes Revising
Causes Chemistry Of Rusting Rust Prevention Introduction To Oxidation Reduction Redox Reactions Gcse Igcse Ks4 Science Chemistry Revision Notes Revising
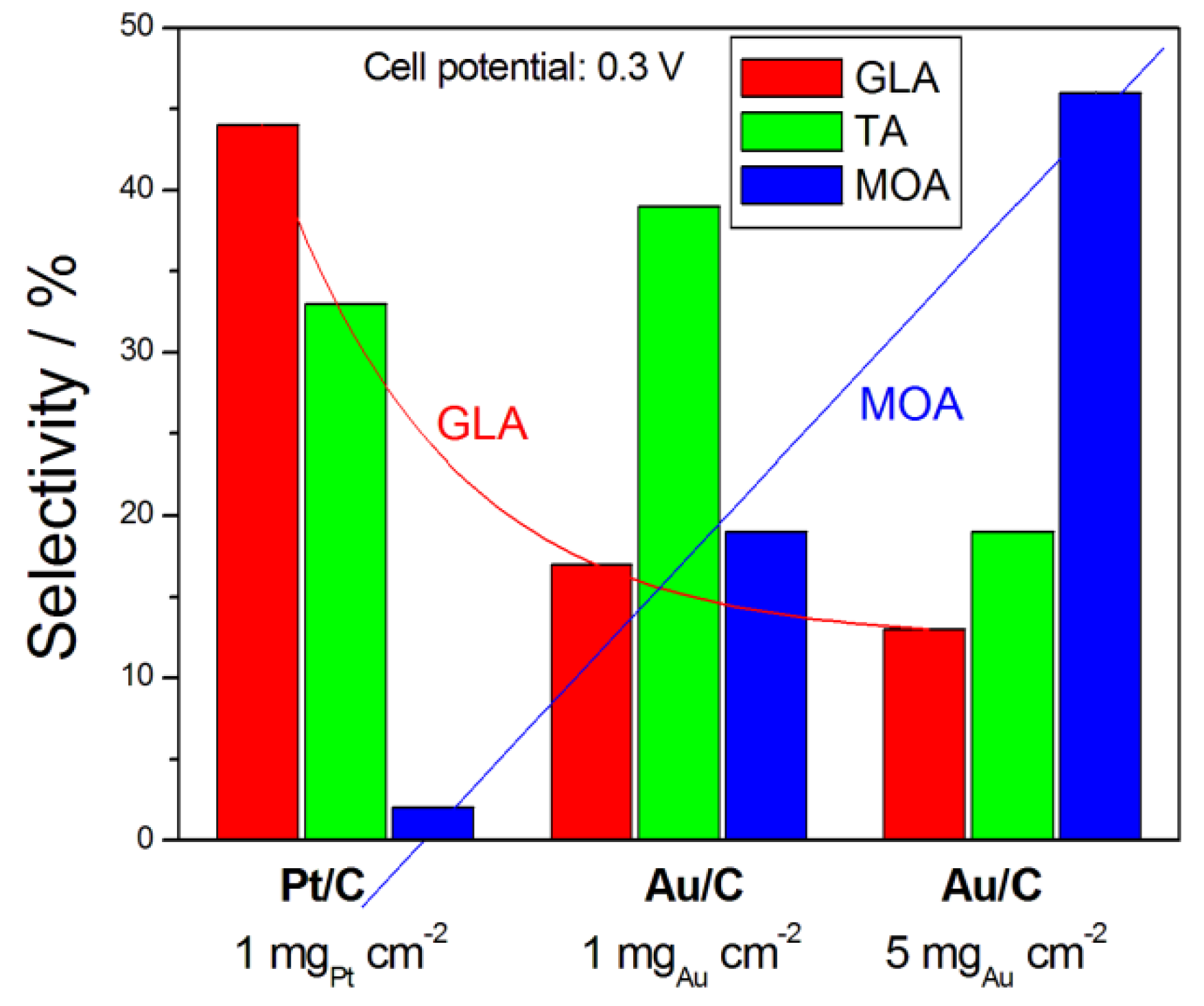
Catalysts Free Full Text Glycerol Electro Oxidation In Alkaline Media And Alkaline Direct Glycerol Fuel Cells Html

How To Oxidize Silver Using Eggs Before And After Silver Oxidized Silver Letterpress Wedding Invitations
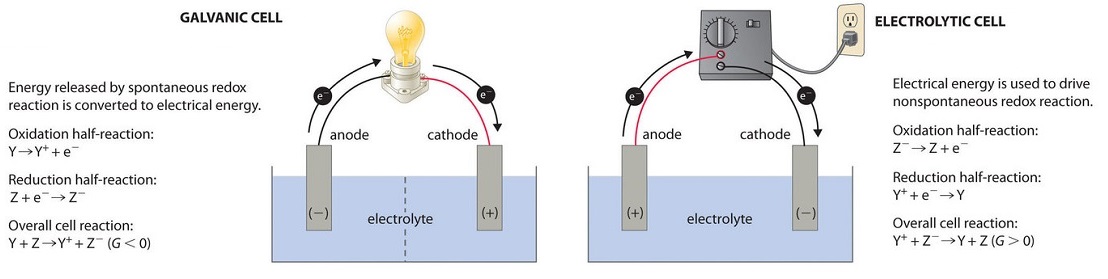
19 3 Voltaic Or Galvanic Cells Generating Electricity From Spontaneous Chemical Reactions Chemistry Libretexts

Why Is Ammonia Nh3 A Weak Base Ppt Download
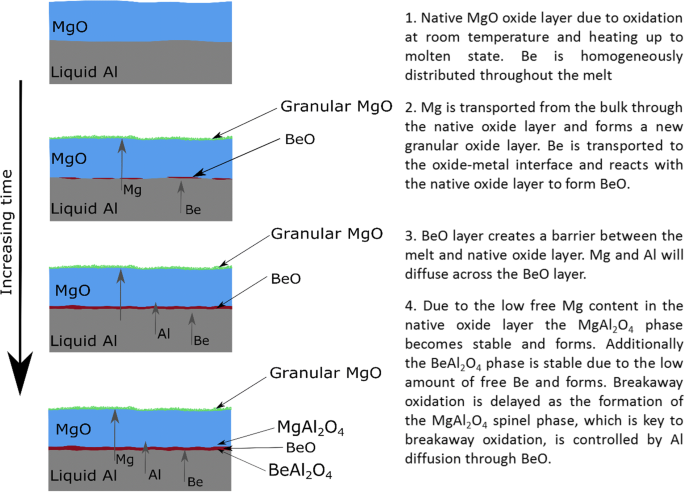
The Mechanism Behind The Oxidation Protection Of High Mg Al Alloys With Beryllium Springerlink
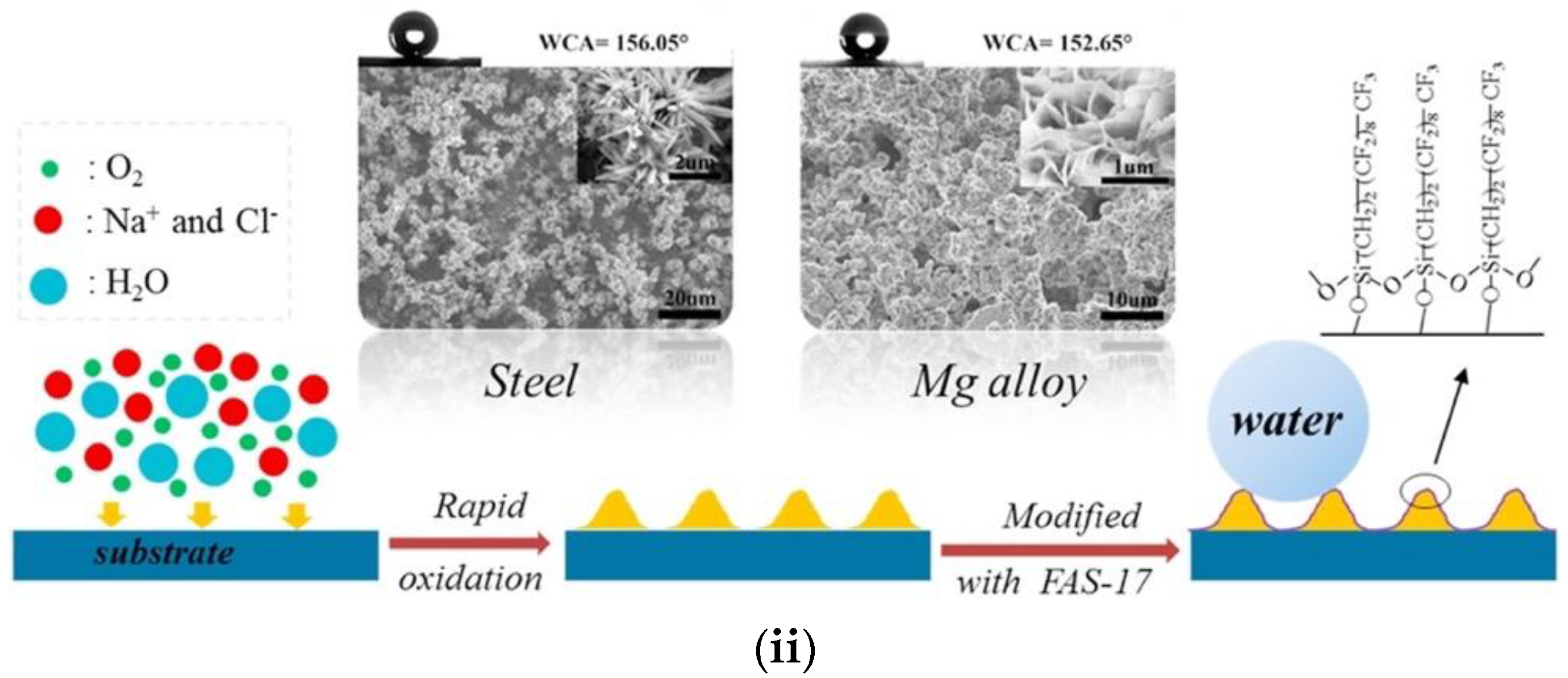
Processes Free Full Text A Review Of Fabrication Methods Properties And Applications Of Superhydrophobic Metals Html
Oxidation Reduction Regents Review Ppt Video Online Download




Comments
Post a Comment